
A new global epidemic has made mRNA vaccine famous, and this innovative medical technology is expected to usher in another breakthrough – cancer treatment. The latest clinical test results in the United States show that mRNA vaccine has proved to be able to deal with the most malignant glioblastoma.
New Altas reported that glioblastoma is one of the most lethal cancer cells, which is most common in brain cancer. The treatment methods include surgical resection, radiotherapy and chemotherapy. Unfortunately, the recurrence rate is very high. Patients can only survive for about one year after diagnosis, and only about 5% of patients survive for more than five years.

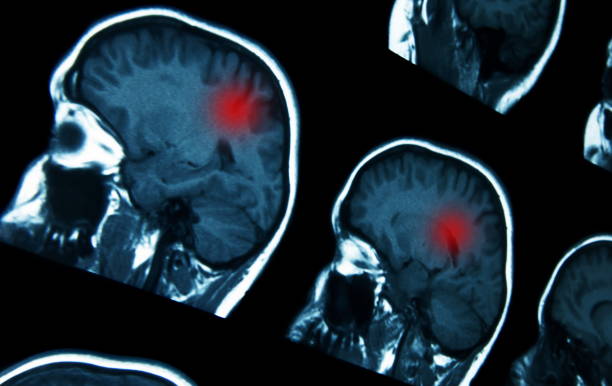
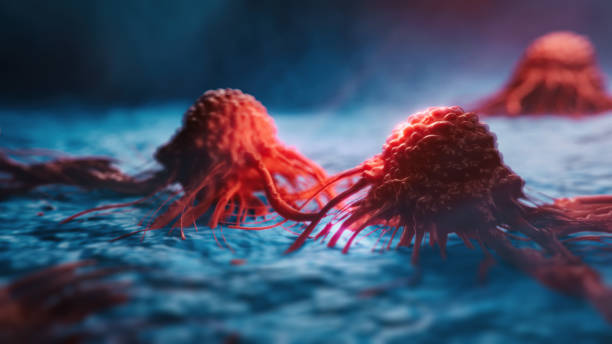
A new study by the University of Florida is expected to soon provide patients with a better choice – the mRNA cancer vaccine. This technology is famous for the COVID-19 vaccine, and has been proven to quickly remind the immune system to attack glioblastomas in mice, dogs and humans more effectively.
The mRNA vaccine breaks the traditional immune activation mode of inactivated and attenuated vaccines, and innovatively uses human cells to produce antigenic bodies to activate specific immune cells. The research team transformed the mRNA vaccine to produce harmless proteins related to pathogens, and the immune system can be trained to resist real pathogens (if it appears).
This small clinical trial approved by the US Food and Drug Administration (FDA) is intended to test the safety and feasibility of only four patients with glioblastoma. After each patient has removed the tumor, RNA is extracted from the tumor, and then mRNA is amplified and wrapped in particle clusters, and then injected into the patient's body to trigger an immune response.
The research team said that it was too early to comprehensively evaluate the clinical effect, but fortunately, the patients did spend a longer disease-free time, and the survival time was longer than expected. In short, mRNA seems to be able to treat brain cancer.
The research team will then expand the scope of the trial to 24 patients to determine the optimal and safe dose. Next, the second phase will involve 25 children.
The study was published in the journal Cell.









+ There are no comments
Add yours